Understanding Anodes for Cathodic Corrosion Protection: A Comprehensive Guide
Corrosion is a naturally occurring process that can cause significant damage to metal structures, leading to costly repairs and replacements. However, with the right protection and preventative methods, we can significantly slow down and halt this process. One such method is cathodic protection, a technique that uses anodes to protect metal structures from corrosion. This post addresses the world of anodes for cathodic corrosion protection, explaining their role, types, and how they work.
Understanding Corrosion
Corrosion is the process by which metals deteriorate due to chemical reactions with their environment. This process can be accelerated by factors such as moisture, oxygen, and salts.
The Role of Anodes in Cathodic Protection
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. This is where anodes come into play. An anode is a sacrificial metal that is more reactive than the metal structure it is protecting. It is connected to the metal structure in an electrical circuit, and due to its higher reactivity, it corrodes instead of the protected metal. This process is known as cathodic protection.
Types of Anodes
There are two main types of anodes used in cathodic protection: galvanic anodes and impressed current anodes.
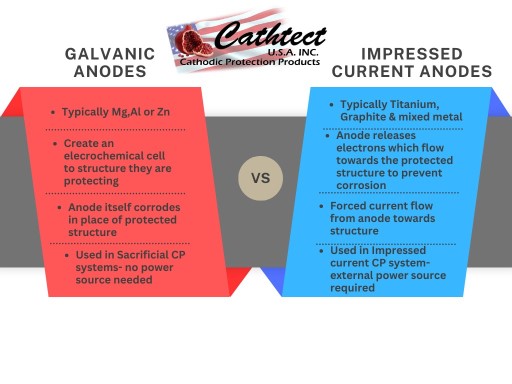
- Galvanic Anodes: These are made from a metal that is more ‘active’ in the electrochemical series than the metal it is protecting. Common materials used for galvanic anodes include zinc, magnesium, and aluminum. These anodes create an electrochemical cell with the protected metal, and they corrode instead of the protected metal.
- Impressed Current Anodes: These are made from a material that is resistant to corrosion, such as titanium & mixed metals or Silicon iron or even graphite. They are used in an impressed current cathodic protection (ICCP) system, where an external power source applies a DC current to the anode. The anode then releases electrons, which flow to the protected metal and prevent it from corroding in a regulated and maintainable design.
How Anodes Work
The working principle of anodes in cathodic protection is based on the electrochemical series, which ranks metals based on their reactivity. When two metals are electrically connected in a conducive environment, the more active metal (anode) will corrode, and the less active metal (cathode) will be protected.
In a galvanic system, the anode is connected to the metal structure, and it corrodes instead of the structure. In an ICCP system, the anode is connected to a DC power source. The power source forces the anode to give up electrons, which then flow to the protected metal, preventing it from corroding.
Anodes play a crucial role in cathodic corrosion protection, offering a cost-effective and efficient method to prolong the life of metal structures. By understanding the structures size, exposure and environment, we can calculate the size and types of anodes and how they will work to protect the structure from the impact of corrosion. We can make informed decisions about the best corrosion protection methods for our Assets and infrastructure design life needs. Whether it’s a ship’s hull, storage tanks, a pipeline, or a steel-reinforced concrete structure, anodes for cathodic protection provide a vital line of defense against the relentless process of corrosion.
Contact our Cathtect USA for for more details on a wide variety of anode options and other Cathodic Protection Products and support @ [email protected] or Call +1 772 646 2873
https://cathtectusa.com/cathodic-protection-products/anodes/?swcfpc=1
Or Request a quote [email protected] and our technical sales team will be happy to answer any questions and assist you in choosing the right anode for all your corrosion protection application.



















